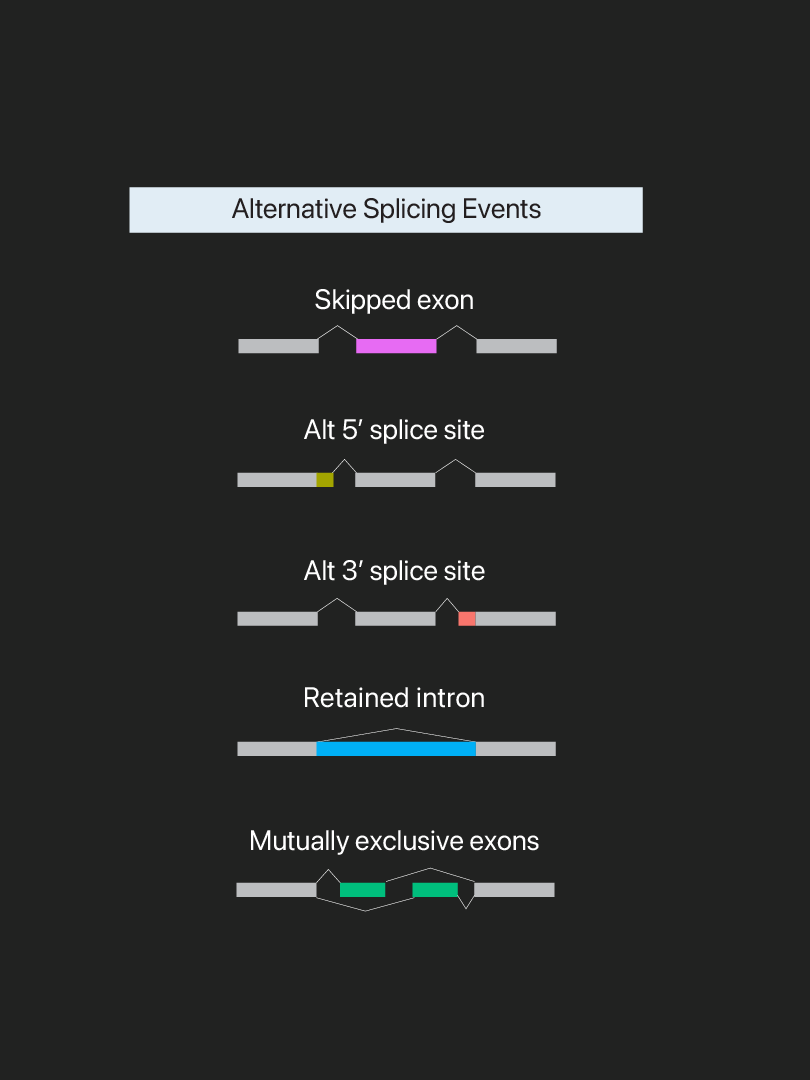
Alternative Splicing
Alternative splicing allows a single gene to code for multiple proteins and greatly expands the diversity of the proteome. It is estimated that up to 95% of human genes undergo alternative splicing. Our genome is said to encompass ~20,400 protein coding genes but can produce up to 100,000 different protein species through alternative splicing. There are seven major types of alternative splicing events: skipped exon, mutually exclusive exons, retained intron, alternative 5′ splice site, alternative 3′ splice site, alternative promotor/first exon, and alternative poly-A site/ terminal exon. The Drosophila Dscam gene represents one of the most extreme cases of alternative splicing. Up to ~38,000 protein isoforms can be encoded through the differential combinations of 95 exons.
Protein vs. RNA
The proteome is sculpted by post-transcriptional forces. A significant portion of protein variance cannot be explained by transcript levels. This non-correlation is best observed in dynamic systems: while an abundant transcript generally produces a more abundant protein over a ow-abundance transcript (high correlation across genes), transcript fold-changes upon stimuli poorly predict those of proteins (low correlation across samples). Some RNA-protein decoupling is traceable to physical constraints or buffering but a majority is due to the many layers of post-transcriptional and post-translational regulations that modify protein levels, including transcript splicing, stability, and translation rates (post-transcriptional) as well as protein localization, modifications, and degradation (post-translational). Because proteins are the primary effector molecules in biology, knowledge into the mechanisms that control protein levels carries fundamental significance to diverse areas of studies.

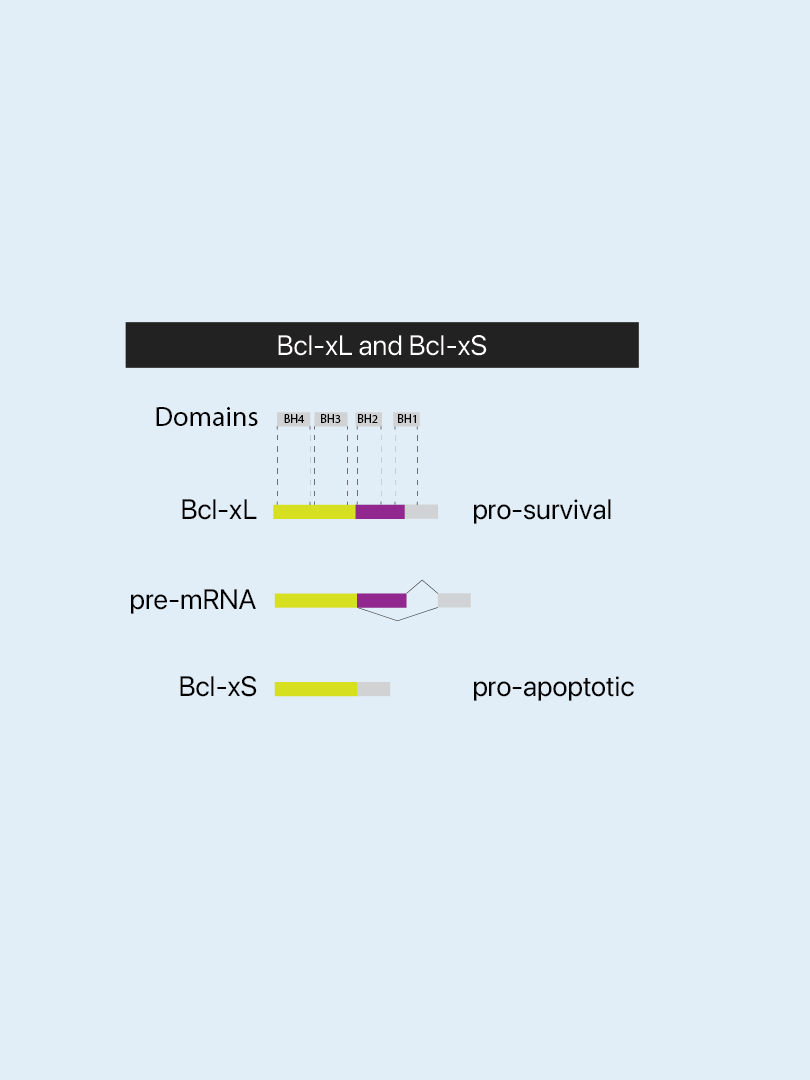
Bcl-xL and Bcl-xS
Both of the proteins Bcl-xL and Bcl-S (depicted above and on the right) are encoded by the BCL2-like 1 gene. They are products of post-transcriptional regulation (i.e., differential splicing at 5′ sites). While xL promotes survival, xS is pro-apoptotic. Apoptosis induction in tumor cells has long been an aim of anti-cancer drugs. Conversely, the prevention of cell death is essential in treatments of cardiac failure. The two Bcl-x isoforms are intensively studied in both disease fields albeit for opposing goals of therapies. This also exemplifies how a delicate balance is necessary between iso-forms from the same genes and that pathologies of different diseases are beneath the surface interconnected.
Maggie Lam Lab
-

Cake First, Worries Later
After weeks of editing, gallons of coffee, and some very intense science moments, our pre-/post-doc AHA grants were submitted this…
-
NHLBI R01 Renewal Application Receives 14th Percentile
We look forward to continuing our work on protein alternative splicing and examine how splice variants affect proteostasis in cardiac…
